The Big Picture
- Not everyone responds to a medication in the same way — for some people, a medication may be effective, while for others, the same medication may cause a harmful reaction or have no effect at all.
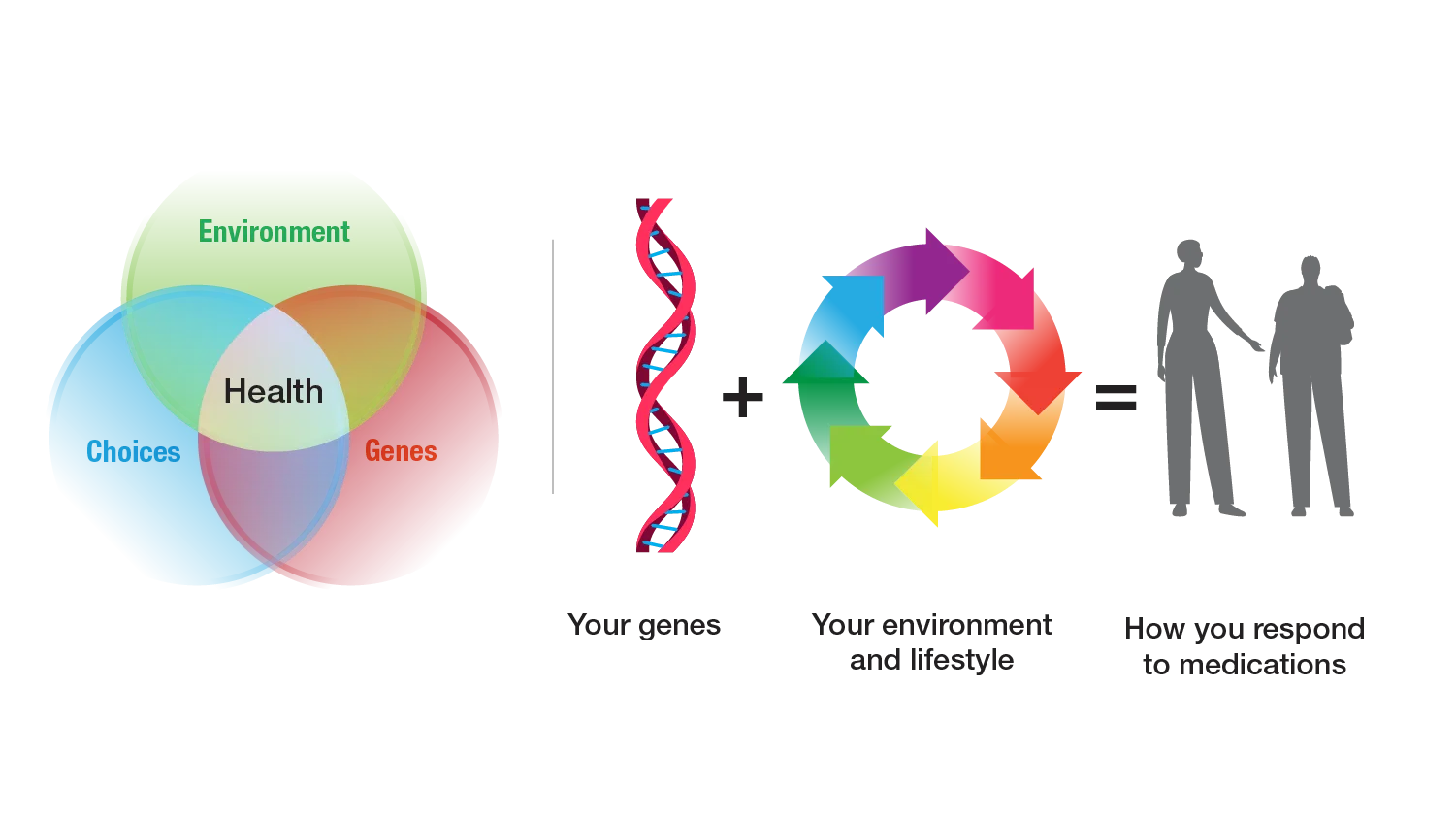
- Multiple factors influence how a person responds to medications, including their genome, environment, lifestyle, and medical history, as well as any other medications they may be taking.
- Most people have genomic variants that could affect how they respond to medications.
- Pharmacogenomic testing involves analyzing a person’s DNA to identify genomic variants that may inform which medication or what dosage of a medication should be prescribed.
What is pharmacogenomics?
Pharmacogenomics combines two scientific areas, pharmacology and genomics, to study how a person’s DNA affects their response to medications. A person’s DNA contains genomic variants that influence how their body handles that medication. Because of this natural genomic variation, people can respond differently to a medication. A gene that is known to contain variants in some people that affect their response to a medication(s) is called a pharmacogene. Pharmacogenomics can help healthcare providers better predict if a medication will be helpful for their patient, what dosage is most appropriate and if a patient is at risk for an adverse reaction.
Pharmacogenomics is part of the growing medical areas of genomic medicine and precision medicine (also called personalized medicine).
What is pharmacogenomic testing?
Studies indicate that more than 98% of people may have a genomic variant that could affect how they respond to commonly prescribed medications. Pharmacogenomic testing can determine whether a patient has specific genomic variants that could affect how they respond to a specific medication. The testing involves analyzing the patient’s DNA obtained from a saliva sample, a blood sample or a cheek swab. Based on the results, the healthcare provider can make more informed recommendations of which medication (typically among a set of options) and dose will be most effective for that patient while minimizing the risk of an adverse reaction.
Who may benefit from pharmacogenomics?
Pharmacogenomic testing may benefit people:
- with new diagnoses or a new medication prescription
- whose medication may not be working
- who are experiencing an adverse reaction or side effect from a medication or have experienced one in the past
- who are taking multiple medications or have other medical conditions that may affect how they respond to medications
- who are taking a medication that is known to be affected by genomics
- who want to be informed about how genomics may affect their response to a medication that they may need in the future
Anyone considering pharmacogenomic testing should talk to their healthcare provider about their current medications and whether such testing is right for them.
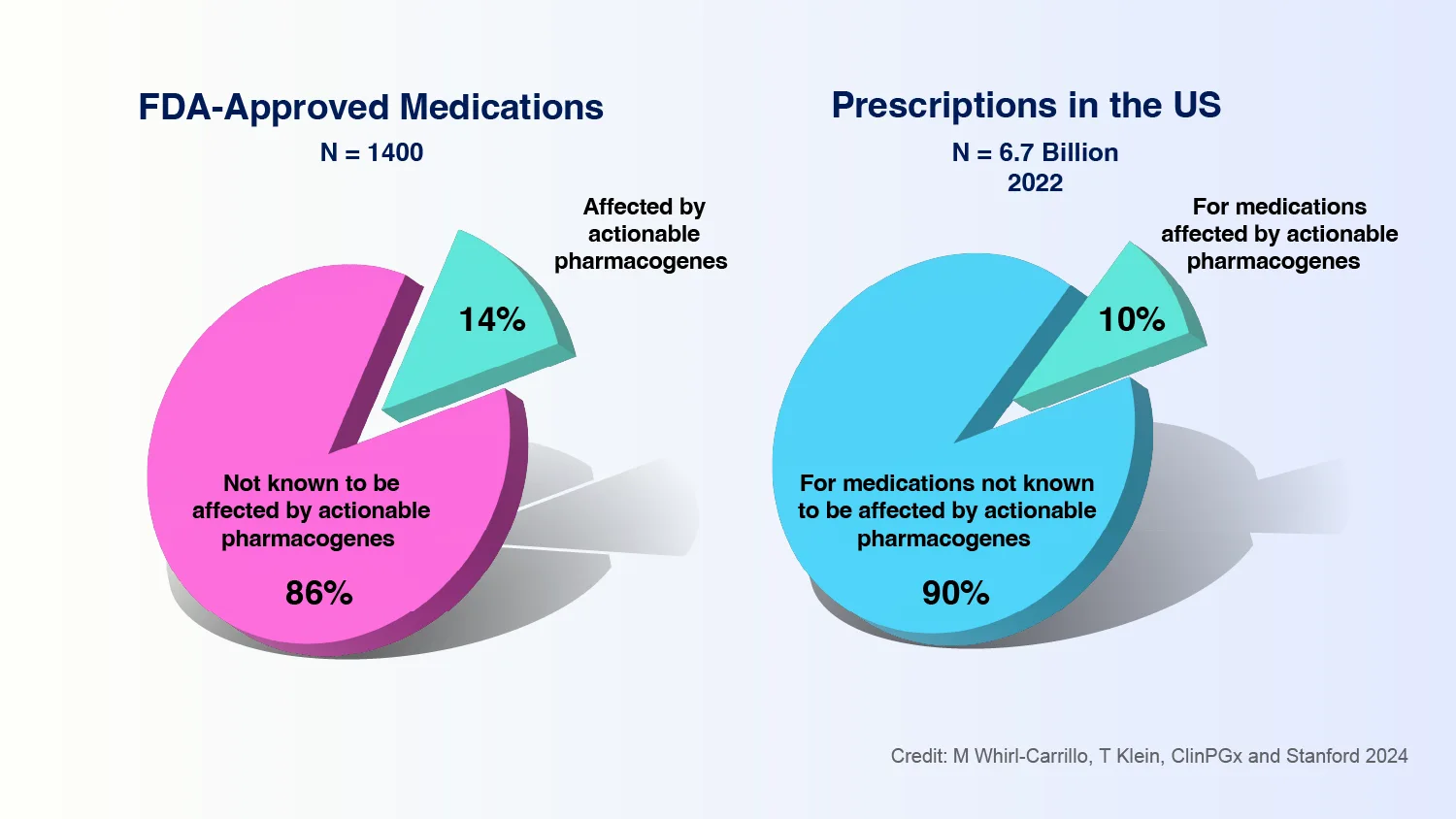
Caption: In 2022, 14% of FDA-approved medications had a pharmacogenomic testing recommendation, affecting 6.7 billion outpatient prescriptions in the U.S.
Genomics affects how a person’s body processes medications
The way in which a person’s body metabolizes, or processes, certain medications can be strongly influenced by genomics. In general, the metabolism of medications is controlled by many proteins, which are encoded by genes. Genes contain the information needed for proteins to be made, activated, and function correctly in the body. Different genomic variants can change the structure of these proteins and influence how they break down or activate certain substances, like medications.
For example, the CYP2D6 gene encodes a protein that is involved in the metabolism of paroxetine (one common brand name of which is Paxil), a medication that is sometimes prescribed for depression. People with certain genomic variants in the CYP2D6 gene can metabolize paroxetine too quickly or too slowly. The medication will not be as effective if metabolized too quickly, and if it is metabolized too slowly, the medication can build up in the body and cause adverse reactions.
Not only does the CYP2D6 gene affect the metabolism of paroxetine, its encoded protein is involved in the metabolism of about 20% of commonly prescribed medications, ranging from antidepressants to pain medications to heart medications.
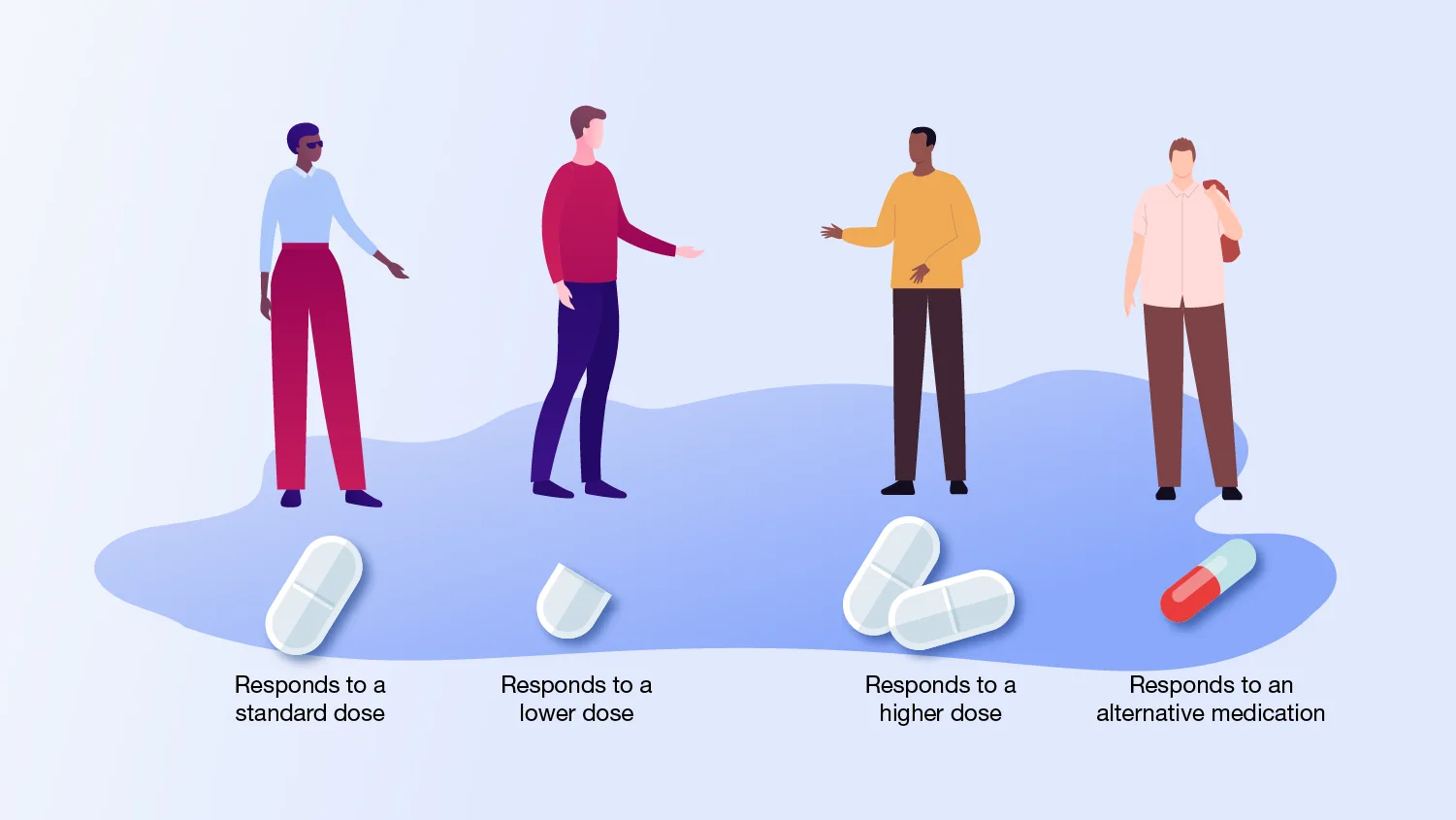
How can genomics predict adverse reactions?
Medications can lead to adverse reactions that may occur in some people but not others. For some medications, genomic variants contributing to these adverse reactions have been identified. More than 6% of hospital admissions are due to adverse drug reactions, and avoiding such adverse reactions using pharmacogenomic testing would be highly beneficial.
One example is simvastatin (one common brand name of which is Zocor), a medication prescribed to patients with high cholesterol. The SLCO1B1 gene encodes a protein that transports this medication into the liver. Some people have genomic variants in the SLCO1B1 gene that alters the protein and slows this transport, causing the drug to build up in the blood and leading to pain and muscle weakness. Healthcare providers can test to see if a patient has one of these genomic variants in the SLCO1B1 gene, and if they do, prescribe a lower dose or a different cholesterol-lowering medication.
In some cases, pharmacogenomic testing can be used to predict immune reactions to certain medications. For example, people who have a specific genomic variant in the HLA-B gene are at a higher risk for a severe allergic reaction to abacavir , which is used to treat HIV, and pharmacogenomic testing can be used to avoid this potentially life-threatening effect.
Genomics can identify medication targets
Some medications are developed to specially target certain cells in the body. Often, this involves the medication interacting with a certain receptor, a protein located on the cell’s surface. The medication needs to fit perfectly onto the receptor, like a lock and a key. Genomic variants within the gene encoding that receptor can influence things like the shape of the receptor or how many receptors on the surface of each cell.
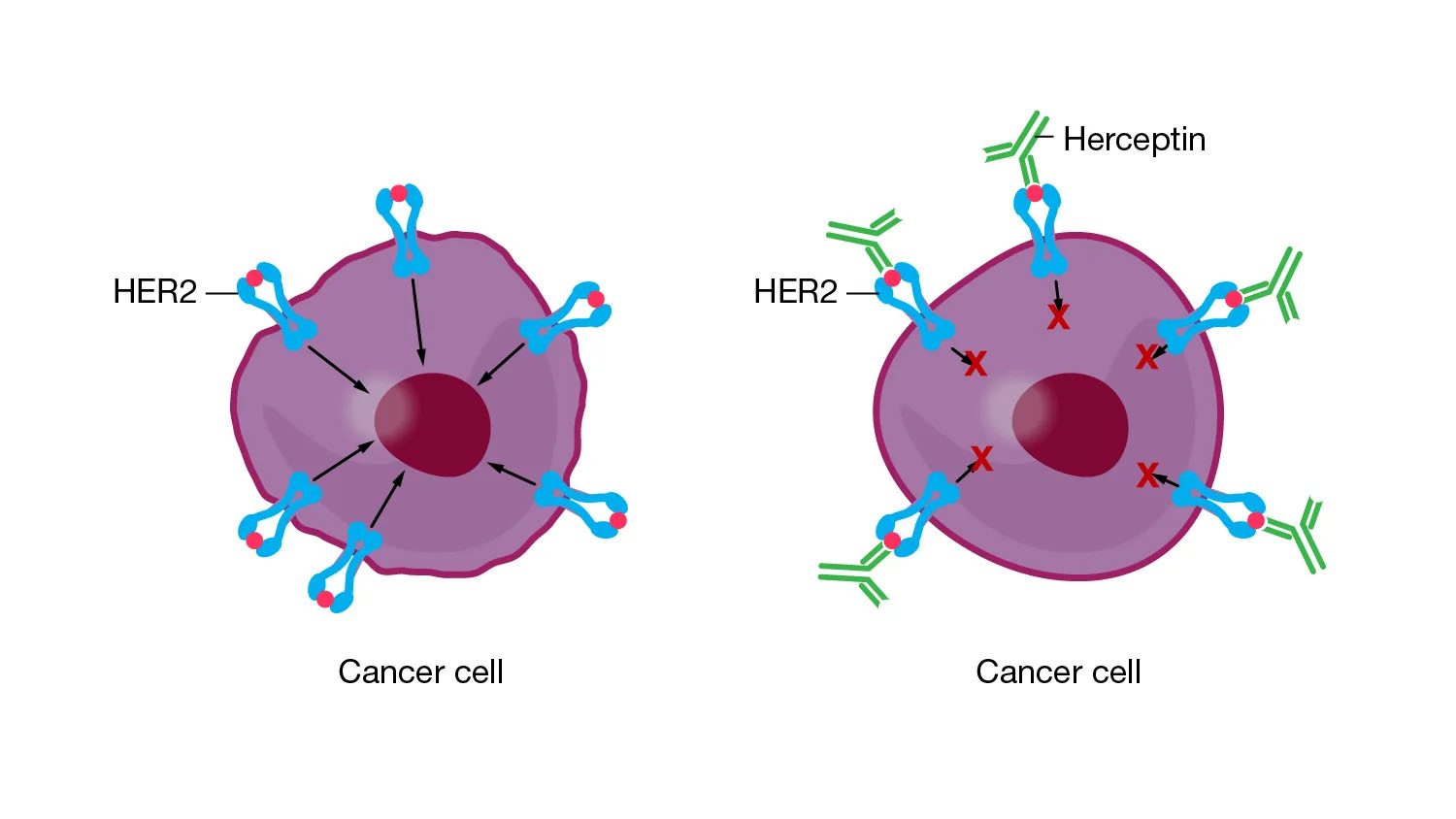
Pharmacogenomic testing can be used to determine if the tumor cells in a cancer patient have a specific version of a receptor, which may influence the choice of treatment. For example, some patients have breast cancers containing a receptor called HER2, which is called HER2-positive breast cancer.
HER2 receptors can cause the cancer to grow and spread more quickly, but the HER2 receptor can also be a target for treatment. The drug trastuzumab (one common brand name of which is Herceptin) attaches to the HER2 receptor, which both blocks the cancer cells from growing and signals the patient’s immune system to kill the cancer cells. Pharmacogenomic testing of a patient’s breast cancer tumor can be used to determine if that tumor contains the HER2 receptor and indicate if trastuzumab would be a good choice for treatment.
What is the future of pharmacogenomics?
It is important to note that pharmacogenomic testing cannot identify the “perfect” medication for a patient. Rather, it uses genomic information about a patient to identify which medications among a list of options are most likely to work, least likely to work or likely to cause an adverse reaction.
Pharmacogenomic testing is not yet available for every medication or every medical condition. However, scientists have identified over 100 medications for which known genomic variants play an important enough role to inform the guidelines for prescribing those medications. Meanwhile, researchers are continuously identifying new interactions between medications and genomic variants, in addition to studying other factors that influence medication response (such as environment, lifestyle, and other medications and medical conditions).
Patient Stories
Pharmacogenomic testing is currently being used in clinical care to inform decisions related to the prescription of medications, and thousands of patients have already benefited. To show the impact of pharmacogenomic testing, we adapted a true patient story from the NIH Clinical Center, omitting the name for privacy reasons.
“I am an African American woman in my 30s and was recently diagnosed with colon cancer. After completing my first cycle of chemotherapy, I experienced severe side effects that resulted in hospitalization and supportive care. My doctor recommended pharmacogenomic testing for genomic variants in the DPYD gene. This gene encodes a protein that is responsible for metabolizing 5-fluorouracil, the specific chemotherapy medication that I was initially prescribed. The test results showed that I had two rare genomic variants in the DYPD gene that were associated with slower metabolism of 5-fluorouracil, and this was causing a harmful buildup of the medication in my blood. Based on these results, my doctor reduced my 5-fluorouracil dose, and I was able to complete the rest of my chemotherapy without severe side effects.”
- NIH Clinical Center Patient
Anyone considering pharmacogenomic testing and how it may be beneficial to them should talk to their healthcare provider.
Last updated: December 24, 2024
