What happens after my protocol is accepted?
How do I know the preliminary results?
After the SGFS has received your data in the form of a .vcf file, we will commence annotation. Each project can vary greatly but typically annotation can take roughly 3-6 months. Upon completion of annotation of all samples in the batch, an SGFS staff member will send the PI a detailed report explaining the process and results of annotation. This report will indicate which samples had a preliminary pathogenic or likely pathogenic secondary finding.

How does the SGFS confirm preliminary results?
If a pathogenic or likely pathogenic (P/LP) variant is identified in one of the ACMG 81 genes, we will confirm this result by Sanger sequencing. In order to accomplish this, we need a new DNA sample from these participants and an order in CRIS for confirmation testing.
Ordering a new DNA sample
We will ask a member of your team to re-identify the coded sample(s) that had a P/LP variant and provide the SGFS team with the name, contact information, and a little bit of background information for each of these participants. An SGFS staff member will reach out to these participants to state that there is a potential genetic finding that your team would like to learn more about and that this will require submission of a new sample. They will FedEx the participant a DNA saliva kit with instructions for saliva collection and return shipping material.
Alternatively, your team is welcome to contact your participants and collect and submit these new DNA samples.
Although saliva is strongly preferred as the source of a new DNA sample, in rare instances a blood sample or previously isolated DNA can be accepted. Direct submission of previously isolated DNA can only be accepted for confirmation of secondary findings if:
2. The new sample was processed in a CLIA certified lab.
Blood will only be accepted if the participant cannot physically spit into the saliva collection tube.
Ordering a confirmation test in CRIS
An ordering clinician from your team should use one of the following two CRIS orders to request confirmation testing in CRIS from a second DNA sample (see screenshot below as well):
2. “Exome-Confirmation, Blood, NHGRI”
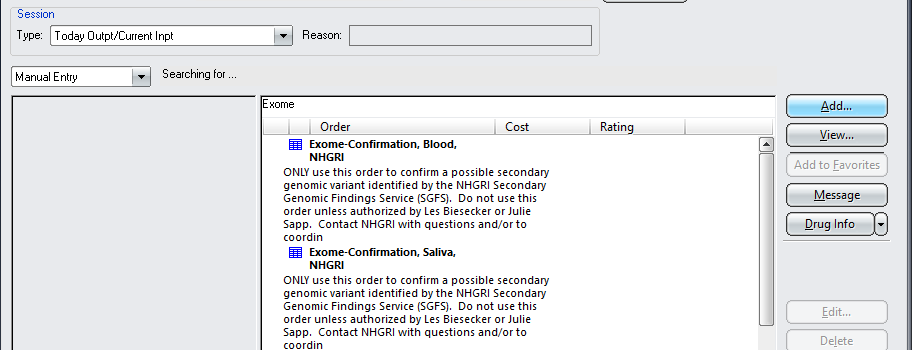
A printout of this order must accompany sample submissions for confirmation testing. If the SGFS is coordinating obtaining a new sample, they can print this order out as long as a member from your team places the order.
Last updated: November 16, 2024

