Gene therapy for sickle cell disease is available to you through FDA-approved therapies (lovotibeglogene autotemcel and exagamglogene autotemcel), and through clinical trials. While there are a number of clinical trials for sickle cell gene therapy, the journey can be similar across all treatments. This is a long process and it is important to understand what happens at each step. Talk with your healthcare team before, during and after each step.
Explore This Page
- Step 1: Referral for Participation in a Clinical Trial
- Step 2: Eligibility
- Step 3: Consent Process
- Step 4: Preparation for Stem Cell Collection
- Step 5: Stem Cell Collection
- Step 6: Manufacturing and Processing
- Step 7: Conditioning and Cell Infusion
- Step 8: Initial Recovery
- Step 9: Continuity of Care
- Step 10: Long-Term Follow-Up

Step 1: Referral for participation in a clinical trial or a treatment
If you are referred to a certain trial, you should learn about:
- Phases of a clinical trial.
- Study outcome(s).
- How researchers will know if the treatment worked.
If you are referred to a certain treatment, you should learn about:
- Phases of the treatment.
- Treatment outcome(s).
- How healthcare providers will know if the treatment worked.
Step 2: Eligibility
Your eligibility to participate in a gene therapy may depend on:
- Your age.
- Your diagnosis of sickle cell disease.
- Your response to other treatments.
- Whether you are taking any other treatments.
- Your overall health.
You may also be required to have:
- Certain screenings (e.g., MRI, blood tests).
- A genetic test to determine if there are changes that would affect your treatment.


Step 3: Consent process
- Read, understand and review the informed consent document. Discuss it with your healthcare team and support system.
- Ask questions. If you still do not understand, either ask again or ask to speak to someone else.
- Know your rights to and the risks of stopping a treatment before it is complete.
Step 4: Preparation for stem cell collection (~90 days)
Gene therapy requires stem cells to be collected from your bone marrow or blood, taken to a lab for modification, then returned to you.
Prior to collection, you may:
- Stop taking hydroxyurea or any other sickle cell disease medication for 2–3 months.
- Receive blood transfusions each month to reduce the number of sickled blood cells.
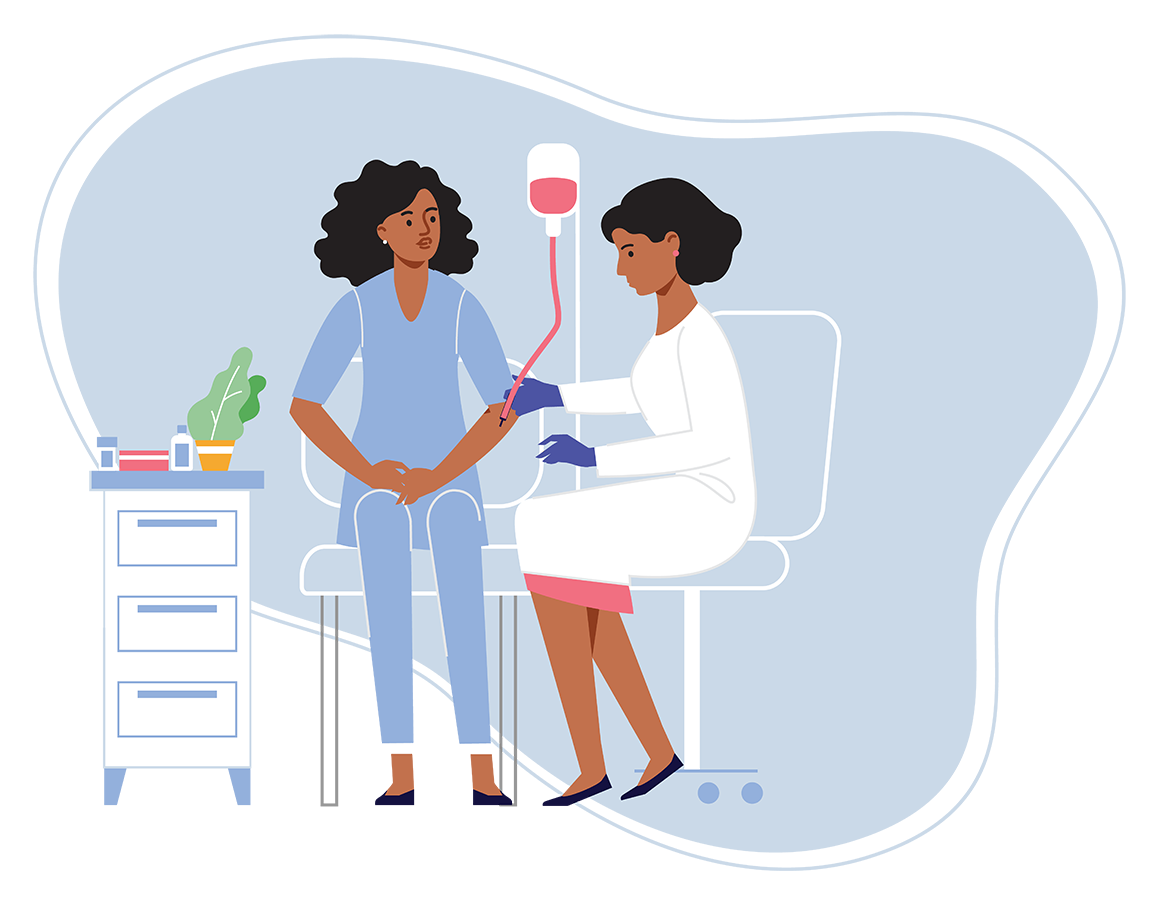
Step 5: Stem cell collection (~6–8 weeks)
The stem cell collection process is called apheresis. Each round occurs over two to three days.
To collect your stem cells, you will:
- Be admitted to the hospital for 1 to 3 days.
- Be given a medication that causes your bone marrow to release stem cells.
- Require an intravenous catheter, which means a catheter will be inserted in your vein.
During collection, stem cells flow into a bag, and the rest of your blood returns to your body.
You may require multiple rounds of the preparation and collection phases to provide enough stem cells. There are usually four weeks between rounds.
Talk with your healthcare team about potential side effects, including pain crisis.
Step 6: Manufacturing and processing
Your collected stem cells will be taken to a special laboratory where they will be modified, or manufactured and processed. This process can take many weeks after your stem cells are collected.
The process differs based on the type of gene therapy approach.
The process does not always work.
- If the process fails, you may need to repeat stem cell collection.
- If the gene-modified stem cells do not meet certain criteria and cannot be returned to your body, then you may not be able to stay in the trial.
If the gene-modified stem cells meet the criteria to be returned to your body, the healthcare team will contact you to schedule your appointment for the next process.
Step 7: Conditioning and cell infusion (~1 week)
To receive your modified stem cells, you will:
- Be admitted to the hospital.
- Receive a chemotherapy drug through an intravenous catheter. This process, called conditioning, will make space in your bone marrow for your modified stem cells.
- Receive your gene-modified stem cells (on Day 0) through a catheter.
Talk with your healthcare team about potential side effects.
Step 8: Initial recovery (~100 days after cell infusion)
Inpatient: 1 month
Because the conditioning process will lower your immune system, you will be moved to a protective room in the hospital to reduce your risk of infection.
Medical staff will monitor you closely through:
- Daily tests (e.g., blood tests, physical exams) to track your progress.
- Medicines to help prevent and treat infections.
Outpatient: 2 to 3 months
Once your healthcare provider determines that your bone marrow and immune system have recovered, you may leave the hospital and move to outpatient care.
Talk with your healthcare team about potential complications.


Step 9: Continuity of care
- You will have a follow-up visit approximately once a month for the first 100 days to see how well the therapy is working, and to track your overall health.
- Your healthcare team should discuss what they will do to help you manage any potential health complications.
Step 10: Long-term follow-up (~15 years)
- Your healthcare team may follow-up with you for 15 years to identify and monitor any long-term effects. This would require a separate informed consent step. It is important to have follow-up visits with your hematology and transplant teams.
- Follow-up visits typically involve annual appointments and bloodwork.
More about sickle cell disease gene therapy
The Democratizing Education Project welcomes your feedback about the sickle cell disease gene therapy resources. Please email your comments or questions to DemocratizingEd@mail.nih.gov.
These educational materials are for informational purposes only. They are meant to promote your general understanding of gene therapy for sickle cell disease. We encourage you to use these educational materials to talk with your healthcare provider or a clinical trial team.
Last updated: September 27, 2024